TAXIS Pharmaceuticals, Inc. is a clinical stage company developing anti-resistance drug candidates that enable the re-use of the most widely prescribed generic antibiotics against antibiotic-resistant ESKAPE pathogens (E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter species). Our TAXISTANCE® anti-resistance drug platform is focused on the disruption of the foundation of bacterial cell wall architecture to address elemental forms of drug resistance. Our focus is on resuscitating the activity of several generic antibiotics, a development that may facilitate access to inexpensive, life-saving medications in community settings across the globe.
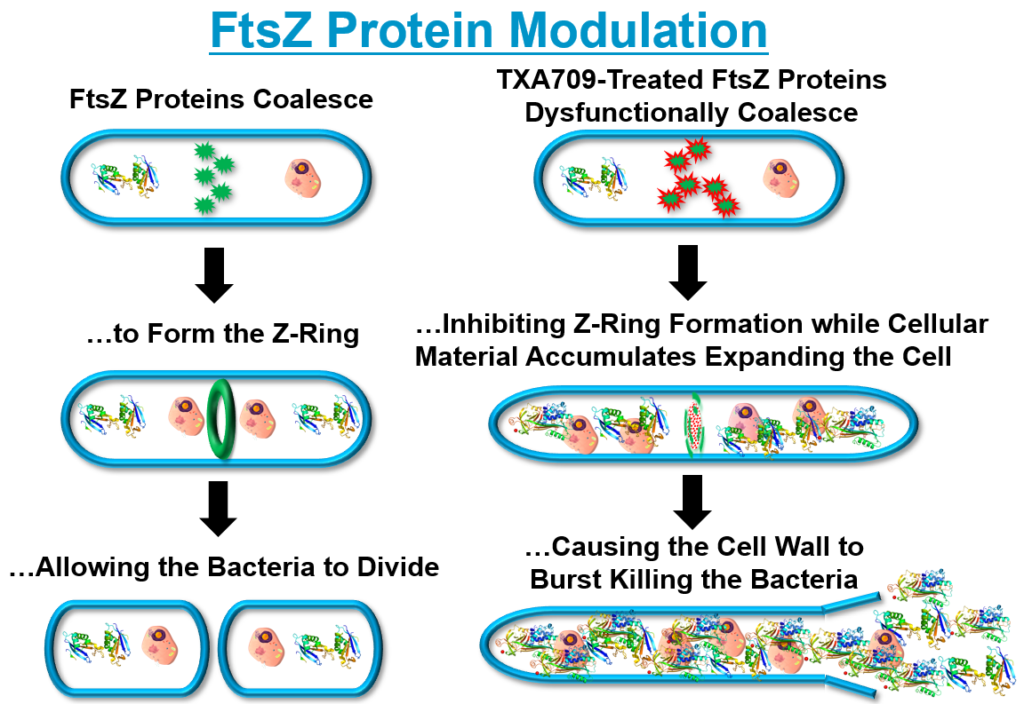
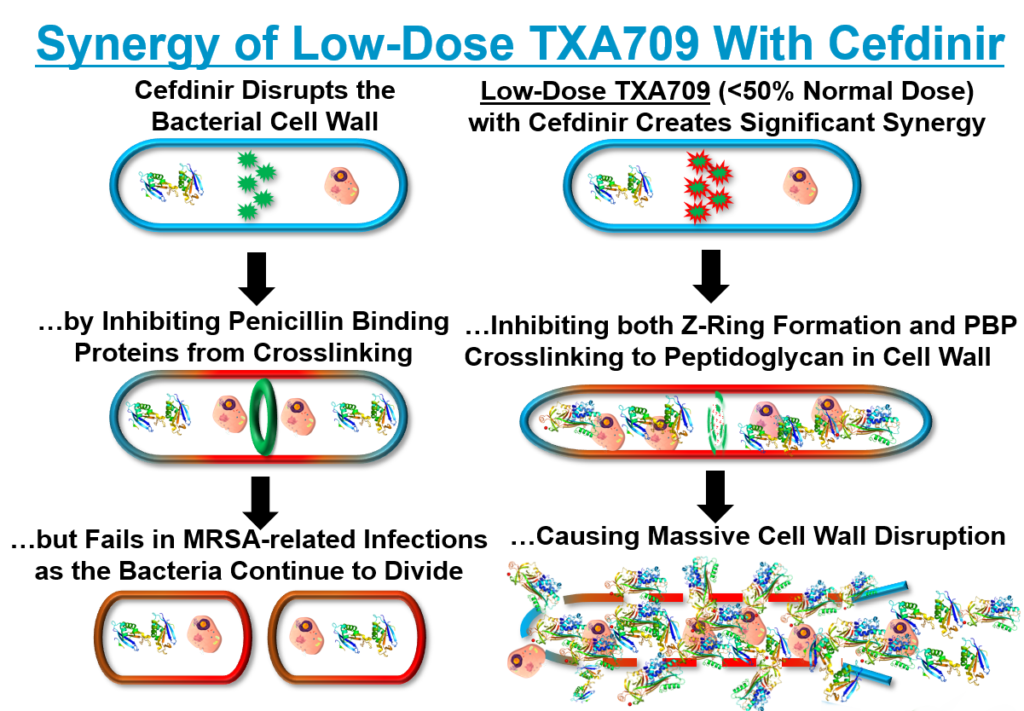
TXA709 FtsZ Modulation
TAXIS’ lead product candidate, TXA709, is an Oral anti-MRSA agent , that recently completed a first-in-human (FIH) Phase I clinical trial with no serious adverse events (SAEs). This is a significant milestone in the evolution of our Company from pre-clinical to development-stage drug discovery. Our ongoing research efforts are focused on disrupting the foundation of bacterial cell wall architecture – including construction, maintenance, and growth – to address elemental drug resistance mechanisms. TXA709 is being developed as an anti-resistance drug to be used in combination with obsolete antibiotics as a fully oral anti-MRSA treatment. TXA709 targets the Filamenting temperature-sensitive mutant Z (FtsZ) bacterial cell division protein. It may also be possible to develop a FtsZ drug candidate targeting Gram-negative bacteria in the future.
In September 2016, the U.S. Food and Drug Administration (FDA) designated TXA709 a Qualified Infectious Disease Product (QIDP), a status that grants TAXIS regulatory incentives including eligibility for fast track designation, priority review, and five additional years of marketing exclusivity. Created under the Generating Antibiotics Incentives Now (GAIN) Act of 2012, the QIDP designation seeks to encourage the development of new antimicrobial drugs to combat the rising threat of multidrug-resistant bacteria.
Efflux Pump Inhibition
Our Efflux Pump Inhibitors (EPIs) represent a new drug class against Gram-negative multidrug-resistant (MDR) pathogens. Bacterial efflux pumps act like bilge pumps by flushing antibiotics out of the bacterial cell and are responsible for antibiotic resistance in many gram-negative strains. TAXIS Pharmaceuticals EPIs have shown that they can resurrect the activity, potency and effectiveness of multiple classes of antibiotics including Macrolides, Cephalosporins, Monobactams, Antimycobacterials, Tetracyclines, Fluoroquinolones and Sulfanomides. Current data reveals synergy with 28 currently approved and marketed antibiotics that no longer work or now require high doses to have any effect.
Efflux, or outward flow, is a process by which foreign substances are moved out of a cell. The process is controlled by transporters called efflux pumps, which are multi-protein complexes that span the bacterial cell membranes. As foreign compounds, such as antibiotics, penetrate the bacterial cell wall, the efflux pumps recognize them and pump them out. As a result, the antibiotics never reach sufficiently high concentrations inside the cell to kill the bacteria, thereby resulting in antibiotic resistance.
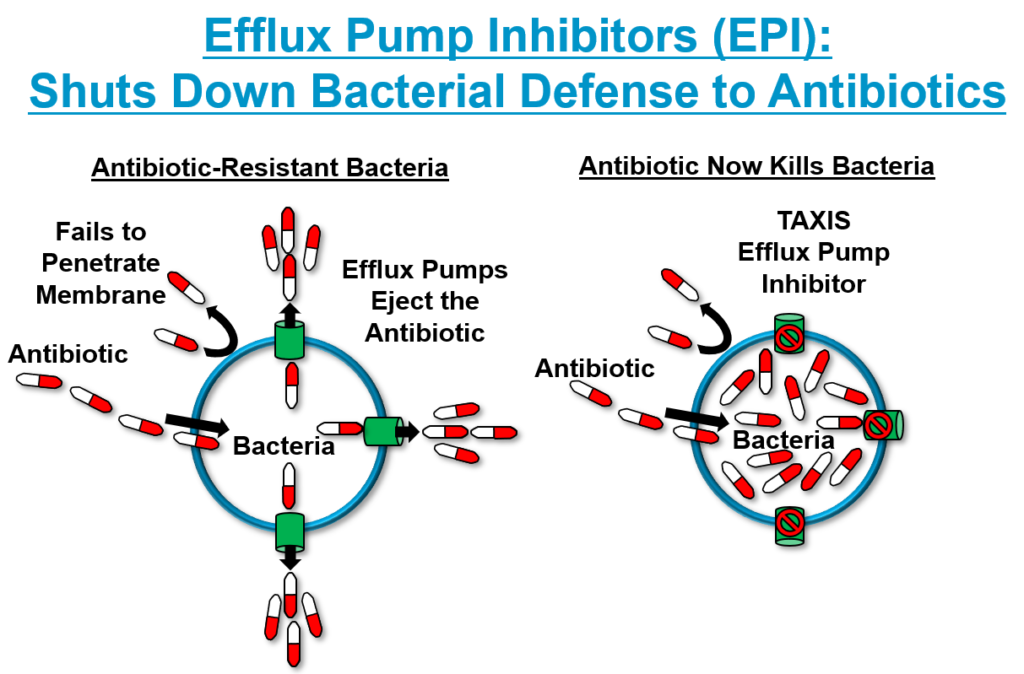
TAXIS is applying the Company’s technology and knowledge toward development of efflux pump inhibitors, or EPIs. This area of research involves reactivation of older, generic antibiotic drugs that are no longer effective because the Gram-negative pathogens they target have developed resistance against them due to the acquisition of efflux pumps.
TAXIS has filed 13 patents arising from our EPI drug discovery program. Since the program’s initiation in 2014, the Company has identified multiple viable EPI candidate compounds, and expects to identify clinical candidates in 2019. The platform, which includes pathogen-specific as well as broad-spectrum EPI drug candidates, impacts a wide range of antibiotic classes, including macrolides, cephalosporins, monobactams, sulfanomides, tetracyclines, and fluoroquinolones. TAXIS’ investigational EPIs have exhibited potent synergy with 28 antibiotics thus far. The Company is developing pathogen-specific EPI candidates that target Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter Baumannii, and Eschericchia coli species; one of these candidates, TXY9155, has demonstrated durable, validated in vivo efficacy in combination with a cephalosporin in a murine septicemia model of P. aeruginosa infection, and appears to potentiate multiple classes of antibiotics against this bacterial species.
TAXIS was recently awarded $3.2M, with potential for an additional $11.4 million, of non-dilutive funding from CARB-X. This Award is a validation of our technology, Team and drug discovery expertise. Our synergistic EPI candidates may similarly revive the commercial prospects of a host of branded antibiotics, enabling the re-introduction of these agents as EPI-based combination products, thereby extending their product lifecycles. Additionally, our synergistic EPI candidates can facilitate research and development efforts by enabling significantly lower antibiotic doses to achieve a therapeutic effect, thereby addressing the problem of dose-related toxicity.
