TAXIS Pharmaceuticals is researching new classes of anti-resistance agents that employ novel mechanisms of action.

TAXIS Pharmaceuticals is researching new classes of anti-resistance agents that employ novel mechanisms of action.
TAXIS Pharmaceuticals is developing three classes of investigational therapies, including:
We are focusing our science on the disruption of the foundation of bacterial cell wall architecture — including construction, maintenance, and growth — to address elemental drug resistance mechanisms. Here are some of the most promising treatments in our current pipeline.
FtsZ Inhibitors
FtsZ inhibitors are a class of compounds that target the bacterial protein FtsZ, which plays a crucial role in cell division. By disrupting FtsZ function, these inhibitors prevent the formation of the bacterial cell’s division machinery, ultimately inhibiting bacterial growth and reproduction. This approach represents a promising strategy for developing new antibiotics to combat.
TXA709 – Our First FtsZ Inhibitor Candidate
- TXA709, TAXIS’ lead product candidate, is a novel target – first-ever product to target FtsZ proteins.
- Oral anti-MRSA agent that completed a Phase I clinical trial with no serious adverse events.
- Ongoing research focuses on disrupting bacterial cell wall architecture to address drug resistance mechanisms.
- TXA709 is developed as an anti-resistance drug for combination therapy with obsolete antibiotics.
- Targets the FtsZ bacterial cell division protein, offering potential for future development against Gram-negative bacteria.
- Designated a Qualified Infectious Disease Product (QIDP) by the FDA in September 2016.
- Regulatory incentives include eligibility for Fast Track designation, priority review, and extended marketing exclusivity under the GAIN Act.

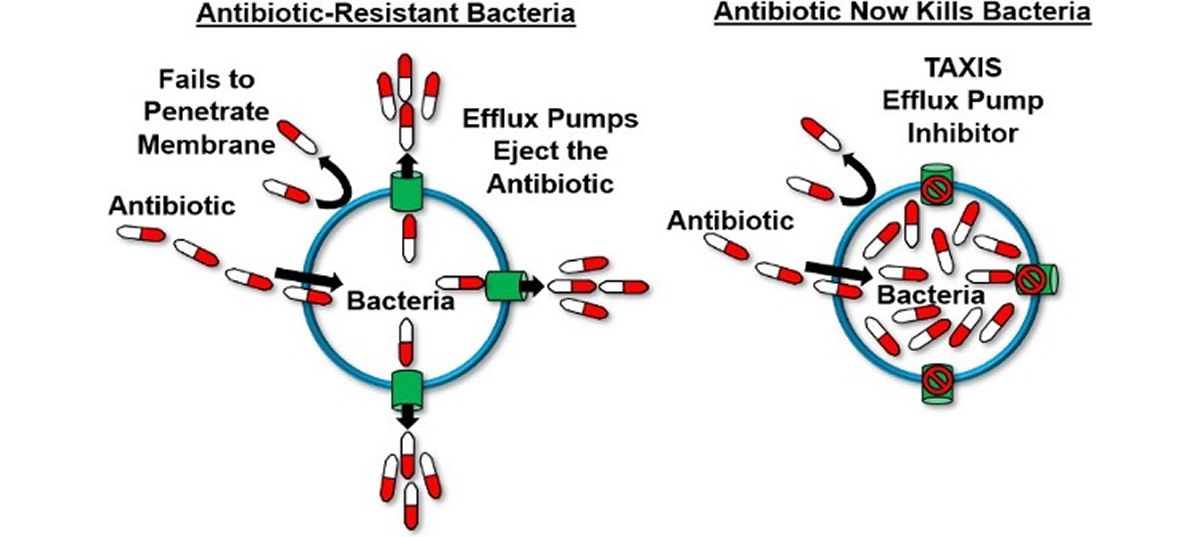
Efflux Pump Inhibitors
Bacterial efflux pumps act like bilge pumps, enabling bacteria to eject a range of compounds, including antimicrobials, from their cells. This ability contributes significantly to antimicrobial resistance and supports bacterial stress responses and virulence, particularly in many Gram-negative bacteria. Efflux pump inhibitors (EPIs) can prevent antibiotics from being expelled, potentially revitalizing the antibacterial drug discovery pipeline.
Using EPIs as adjuvants offers several advantages: they can restore the effectiveness of older antimicrobials, minimize the development of bacterial resistance, and lessen the need for new antibiotic discovery, saving time, effort, and resources. Despite their promise, previous EPI classes have faced toxicity issues that hindered their clinical application, limiting their use mainly to epidemiological studies.
Our novel EPIs stand out as promising first-in-class combination therapies for Hospital-Acquired Pneumonia (HAP) and Ventilator-Associated Pneumonia (VAP) due to several key features:
- Efflux Pumps (EPI’s) serve as bilge pumps, ejecting antibiotics from bacterial cells and contributing to antibiotic resistance.
- EPIs block these pumps, preventing antibiotics from being expelled.
- Our EPIs represent a novel drug class designed specifically for Gram-negative multi-drug resistant (MDR) pathogens.
- They have shown potential to restore the activity and effectiveness of various classes of antibiotics.
- This restoration increases treatment options against resistant infections.
- Our EPIs have received Qualified Infectious Disease Product (QIDP) designation, highlighting their clinical significance.
- The QIDP designation supports expedited development and regulatory benefits for these promising therapies.
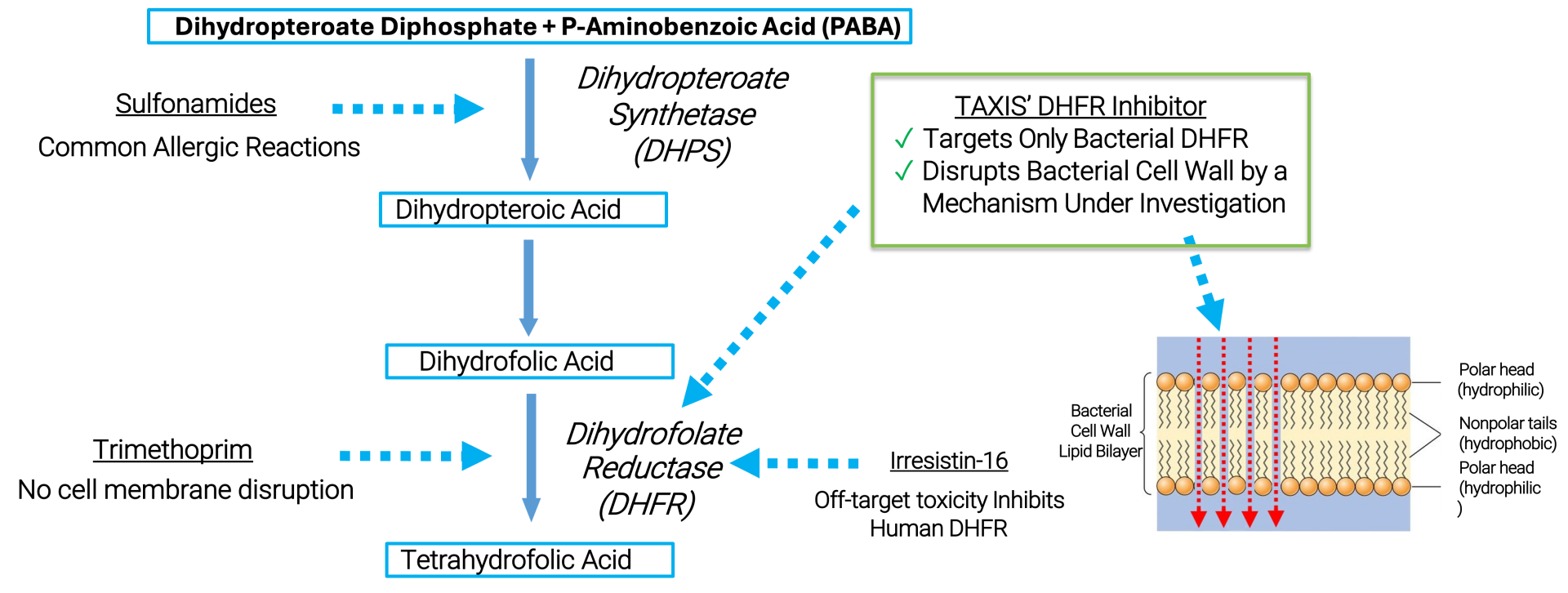
Dihydrofolate Reductase Inhibitors (DHFRIs)
The clinical success of trimethoprim (TMP) demonstrates the potential of targeting the bacterial Dihydrofolate Reductase (baDHFR) enzyme. However, bacterial DHFR inhibitors have not been effective against Neisseria gonorrhoeae (Ng) because of its natural resistance to TMP, which is caused by a reduced affinity of the enzyme for the drug.
With existing antibiotics proving ineffective and the rise of ceftriaxone-resistant strains threatening the last line of defense, Ng has been designated as a priority pathogen by the World Health Organization (WHO).
TAXIS DHFR inhibitors (TDHFRIs) represent a promising new class of drugs that can address multi-drug-resistant gonorrhea infections. They offer several key advantages:
- With existing antibiotics proving ineffective and the rise of ceftriaxone-resistant strains threatening the last line of defense, Ng has been designated as a priority pathogen by the World Health Organization (WHO).
- TAXIS DHFR inhibitors (TDHFRIs) represent a promising new class of drugs that can address multi-drug-resistant gonorrhea infections. They offer several key advantages: